Beta-Blockers: How Different Types Affect Your Heart and Body

Beta-Blocker Selection Tool
This tool helps you understand which beta-blocker type might be most appropriate for your specific situation based on the article content. Select your conditions to receive personalized recommendations.
Your Health Profile
When your heart races from stress, anxiety, or a heart condition, beta-blockers step in to slow it down. But not all beta-blockers are the same. Some target only the heart. Others also open blood vessels. Some cause fatigue. Others might help with sexual function. Choosing the right one isn’t just about the diagnosis-it’s about your body, your other conditions, and even your lifestyle.
How Beta-Blockers Actually Work
Beta-blockers don’t just calm your heart. They block the effects of adrenaline and noradrenaline-the chemicals your body releases when you’re stressed, scared, or exercising hard. These chemicals bind to beta receptors, which then tell your heart to beat faster and harder. Beta-blockers sit in those receptor spots like a lock and key that doesn’t turn. No signal gets through. So your heart rate drops. Your blood pressure falls. Your heart doesn’t work as hard.
This is why they’re used after a heart attack, for irregular heart rhythms, and in heart failure. But here’s the catch: not all beta receptors are the same. There are three main types-beta-1, beta-2, and beta-3-and each plays a different role. Beta-1 receptors are mostly in the heart. Beta-2 are in your lungs, blood vessels, and muscles. Beta-3 are involved in fat breakdown and blood vessel relaxation.
When a drug blocks beta-1 only, it leaves your lungs and muscles alone. That’s why some beta-blockers are safer for people with asthma. Others? They can trigger breathing trouble. And that’s why the class you’re on matters more than you might think.
First-Generation: The Old Guard
Propranolol was the first beta-blocker ever used in medicine. Introduced in the 1960s, it’s still around today. Why? Because it’s cheap. But it’s also nonselective. That means it blocks beta-1 and beta-2 receptors. That’s a problem if you have asthma or COPD. Blocking beta-2 in your airways can cause bronchospasm-your lungs tighten up. It’s rare, but serious.
Propranolol also crosses the blood-brain barrier. That’s why some people on it report depression, vivid dreams, or trouble sleeping. It’s not just a heart pill. It’s a full-body system changer. And because it’s taken twice daily, people often forget doses. Miss a pill? Your heart rate spikes. That’s why many doctors now avoid it unless absolutely necessary.
Another first-gen drug is sotalol, which also has antiarrhythmic properties. But it’s trickier to use. It can cause a dangerous heart rhythm called torsades de pointes if your potassium is low or your kidneys aren’t working well. That’s why it’s usually only given in hospitals or under close monitoring.
Second-Generation: Heart-Selective and Simpler
These are the workhorses of modern cardiology. Drugs like metoprolol, atenolol, bisoprolol, and esmolol were designed to stick mostly to beta-1 receptors. That means they leave your lungs alone. For someone with asthma or COPD, this is a game-changer.
Metoprolol comes in two forms: tartrate and succinate. Tartrate is taken twice a day. Succinate is extended-release-once daily. That’s huge for adherence. A 2022 Cleveland Clinic survey found patients on once-daily metoprolol succinate were 40% more likely to stick with their meds than those on twice-daily tartrate.
Bisoprolol is even more selective. It’s got a long half-life and is mostly cleared by the liver, not the kidneys. That makes it a top pick for older adults or those with kidney disease. Atenolol? It’s cheap and effective, but it’s cleared by the kidneys. If your kidney function drops, the drug builds up. That can lead to too-slow heart rates or low blood pressure. Many U.S. guidelines now discourage atenolol for heart failure because it doesn’t reduce death risk like the others.
Here’s the reality: second-gen beta-blockers cut heart attack deaths by 25% after a heart attack. But they don’t all do the same thing for heart failure. Only some are proven to improve survival there.
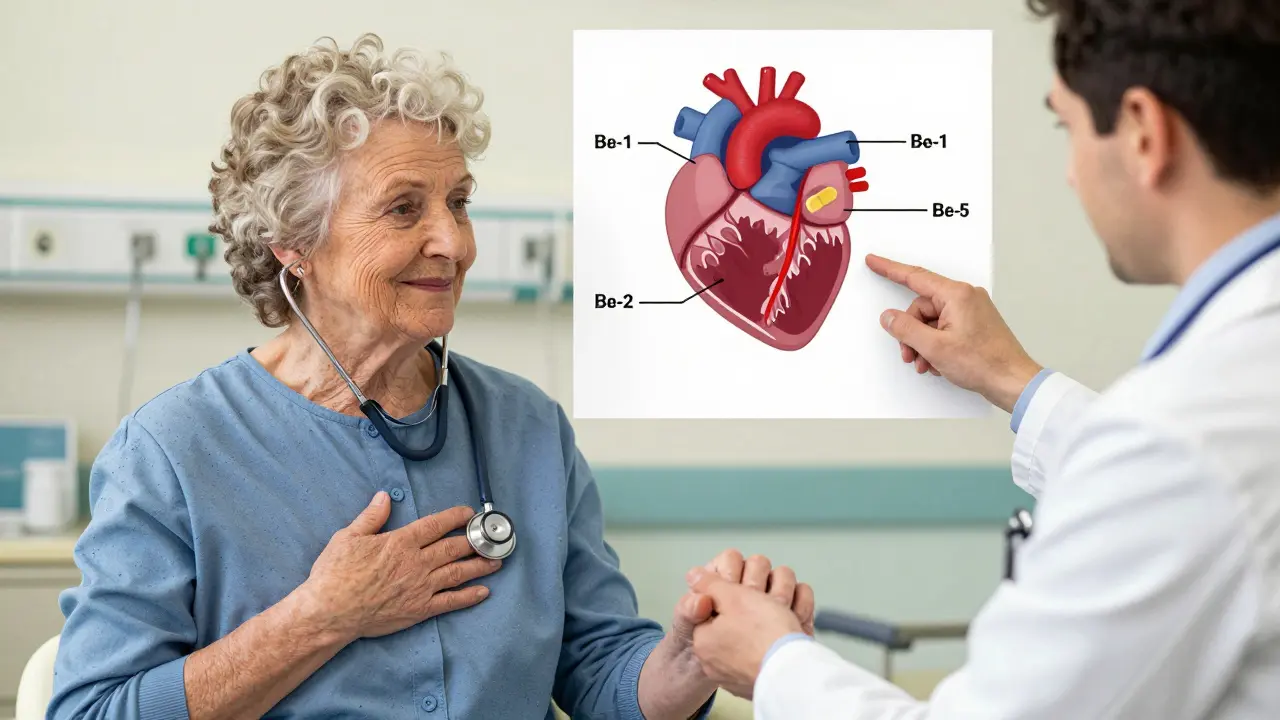
Third-Generation: The Game Changers
This is where things get interesting. Carvedilol and nebivolol don’t just block beta receptors. They do extra things that make them uniquely powerful.
Carvedilol blocks beta-1 and beta-2 receptors-but it also blocks alpha-1 receptors. That’s a vasodilator. It opens up your blood vessels. So while it slows your heart, it also lowers resistance in your arteries. That’s why in the landmark U.S. Carvedilol Heart Failure Study (1996), it cut death risk by 35% compared to placebo. That’s not just better than older beta-blockers. It’s better than most drugs in heart failure.
Then there’s nebivolol. It blocks beta-1 receptors and activates beta-3 receptors. That triggers nitric oxide release-the same molecule that makes your blood vessels relax. It’s why nebivolol lowers blood pressure more than other beta-blockers. It’s also why some men on nebivolol report better sexual function. A Reddit thread from 2023 showed 65% of men over 50 on nebivolol said their libido improved. On traditional beta-blockers? Only 35% saw the same benefit.
Both carvedilol and nebivolol have antioxidant effects. They reduce oxidative stress in heart cells by 30-40%. That means less scarring, less stiffening, less remodeling. In heart failure, that’s huge. The European Society of Cardiology now recommends them as first-line for heart failure with reduced ejection fraction. Not just because they slow the heart-but because they protect it.
Why Some People Can’t Tolerate Beta-Blockers
Side effects aren’t random. They’re tied to the drug’s chemistry.
Metoprolol and propranolol can cause fatigue in 40% of users. Why? Because they lower heart rate too much, especially at rest. If you’re active, your body can’t ramp up. You feel sluggish.
Cold hands and feet? That’s beta-2 blockade reducing blood flow to your extremities. Nonselective drugs like propranolol do this more. Selective ones like bisoprolol? Less so.
Depression and sleep issues? Linked to drugs that cross into the brain. Propranolol does. Nebivolol? Doesn’t. That’s why nebivolol has a 7.1/10 rating on Drugs.com, while propranolol sits at 6.2/10.
And then there’s the risk of sudden withdrawal. If you stop beta-blockers cold, your body goes into overdrive. Adrenaline surges. Heart rate spikes. Blood pressure rockets. The risk of heart attack in the first 48 hours? Up 300%. That’s why doctors always taper them-over days or weeks. Never quit on your own.

Who Gets Which Beta-Blocker?
There’s no one-size-fits-all. Here’s how it breaks down:
- After a heart attack? Bisoprolol, carvedilol, or metoprolol succinate. All proven to save lives.
- Heart failure with reduced ejection fraction? Carvedilol or nebivolol. They do more than slow the heart-they heal it.
- Asthma or COPD? Avoid nonselective drugs. Use bisoprolol or nebivolol. Even then, start low and go slow.
- High blood pressure alone? Beta-blockers are no longer first choice. ACE inhibitors, ARBs, or calcium channel blockers work better. But if you have another condition-like migraines or tremors-beta-blockers still win.
- Older adults? Bisoprolol or nebivolol. Less kidney strain. Fewer side effects.
- Men with erectile dysfunction? Nebivolol. It’s the only one linked to improved sexual function.
The American College of Cardiology has a free toolkit with 12 factors to help choose: kidney function, liver health, diabetes, sleep issues, activity level, other meds, age, gender, symptoms, lung disease, heart rhythm, and prior side effects. It’s not guesswork. It’s precision.
The Future of Beta-Blockers
In 2023, the FDA approved a new drug-entricarone-that combines a beta-3 agonist with a beta-1 blocker. It’s for heart failure with preserved ejection fraction, a condition with no good treatments. Early trials show 22% fewer hospital stays.
Another combo-nebivolol with valsartan-is coming in 2024. It’s like hitting two birds with one stone: block adrenaline and relax blood vessels.
And researchers are testing gene-based selection. If your DNA shows you metabolize beta-blockers slowly? You’ll get a lower dose. If your genes make you prone to fatigue? Skip propranolol. This isn’t sci-fi. The GENETIC-BB trial is underway.
By 2028, third-generation beta-blockers will make up 60% of prescriptions. Why? Because they don’t just treat symptoms. They change outcomes. They save lives.
So if you’re on a beta-blocker, ask: Is this the right one for me? Not just because your doctor prescribed it. But because your body deserves more than a one-size-fits-all pill.
Tasha Lake
February 7, 2026 AT 08:05Okay, so let me get this straight-beta-1 selectivity isn’t just about avoiding bronchospasm, it’s about preserving metabolic flexibility too? That’s wild. I’ve been on bisoprolol for AFib and never realized how much it spared my peripheral vasculature. My hands aren’t ice cubes anymore, and my resting HR’s stable without me feeling like a zombie. The fact that nebivolol triggers NO release? That’s not just pharmacology-that’s biomolecular poetry.
Simon Critchley
February 7, 2026 AT 14:03LOL so propranolol’s the OG beta-blocker and now it’s basically the villain in a superhero movie? 😂 I remember my doc trying to switch me off it because I’d get nightmares and feel like I was underwater all day. Turns out crossing the BBB = brain drama. Also, 65% of men on nebivolol report better sex? Bro, that’s the only reason I’d switch. My wife’s been hinting for years. 💯