Cancer Chemotherapy Safety: How to Handle and Administer Antineoplastic Drugs Correctly
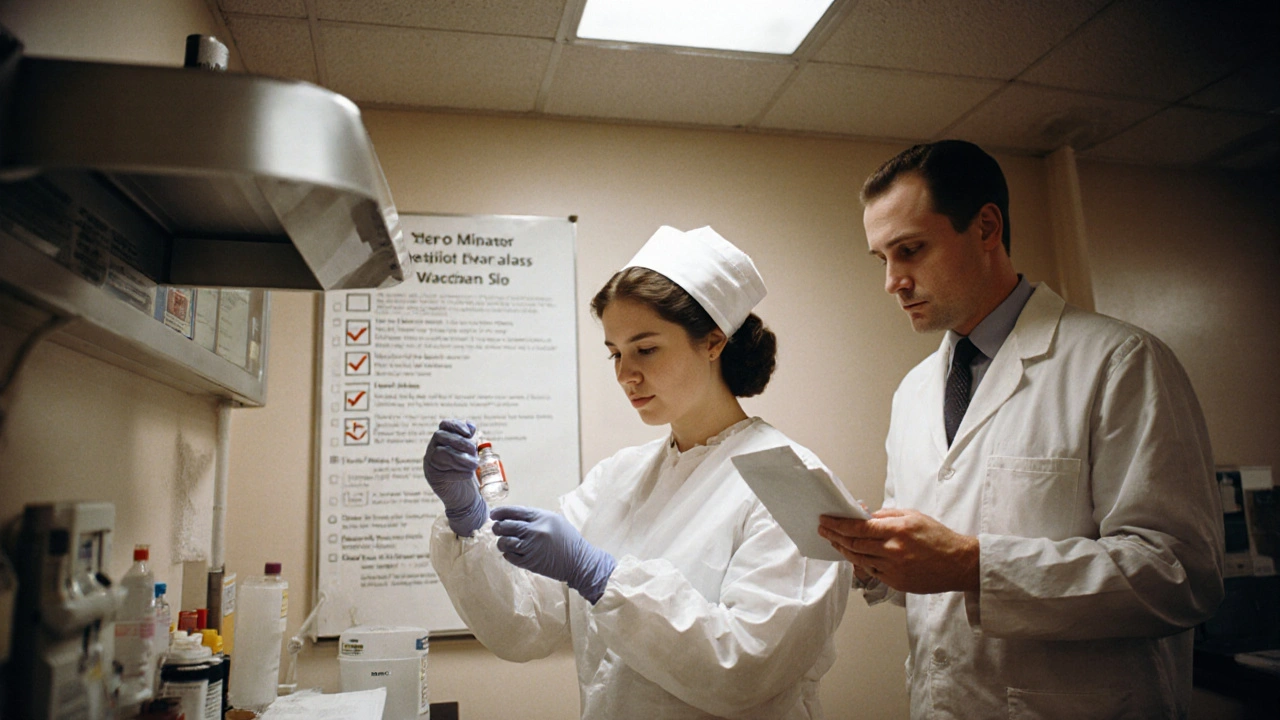
Handling cancer chemotherapy isn’t just about giving a drug. It’s about protecting lives - the patient’s, the nurse’s, even the caregiver’s at home. These drugs don’t just kill cancer cells. They can damage healthy tissue, cause long-term harm to staff, and contaminate surfaces if not handled right. A single mistake - a torn glove, a missed verification, a spilled vial - can have serious consequences. The good news? We know how to prevent most of these errors. The bad news? Too many places still cut corners.
Why Chemotherapy Is Different From Other Medications
Most pills or injections you take at home are designed to be safe for anyone to handle. Chemotherapy drugs are not. These are cytotoxic agents - they’re built to destroy rapidly dividing cells. That’s why they work against cancer. But they don’t know the difference between a tumor and your hair follicles, bone marrow, or digestive tract. Even tiny amounts of exposure over time can lead to infertility, nerve damage, or increased cancer risk for healthcare workers.
That’s why the rules changed. In 2024, the American Society of Clinical Oncology (ASCO) and Oncology Nursing Society (ONS) updated their safety standards to reflect modern oncology. They stopped calling it just “chemotherapy.” Now it’s “antineoplastic therapy,” because treatments include targeted drugs, immunotherapies, and antibody-drug conjugates - all of which carry similar risks. Some, like carmustine and thiotepa, can penetrate gloves in under 10 minutes. Others can become airborne during preparation. This isn’t theoretical. Studies from 1992 to today show contamination spreads from gloves to skin, to countertops, to children’s toys.
What Personal Protective Equipment (PPE) You Really Need
Wearing gloves isn’t enough. You need the right gloves. Regular latex or nitrile gloves? They won’t cut it. You need chemotherapy-tested double gloves - specifically designed and tested to resist permeation by hazardous drugs. The National Institute for Occupational Safety and Health (NIOSH) has clear guidelines: two pairs, changed every 30 minutes or immediately if torn. And it’s not just gloves.
You need an impermeable gown - not a regular lab coat. Eye protection if there’s any chance of splashing. A mask or respirator if you’re opening vials, reconstituting powders, or dealing with spills. And here’s something most people don’t realize: once you’ve handled chemotherapy, your PPE is contaminated. Even if it looks clean. You can’t reuse it. You can’t wash it. You can’t just toss it in the regular trash. It goes into a special hazardous waste container labeled for antineoplastic agents.
Facilities that skip any part of this - say, skipping eye protection because “it’s just a small dose” - are gambling with staff health. A 2022 survey found that 78% of oncology nurses who followed full PPE protocols reported feeling more confident. Those who didn’t? Many had experienced skin rashes, headaches, or nausea after handling drugs.
The Four-Step Verification Process: Your Last Line of Defense
The biggest change in the 2024 standards is the mandatory fourth verification. Before, you had two people check the drug, dose, and patient. Now, you need a third person to check again - and then a fourth person does it right at the patient’s bedside, just before the infusion starts.
This isn’t bureaucracy. It’s survival. In 2022, the National Comprehensive Cancer Network (NCCN) database showed that 18% of chemotherapy-related errors were due to patient misidentification. Someone got the wrong drug because their name sounded like another patient’s. Or their chart was mixed up. Or they were in Room 3 instead of Room 5. The fourth check forces a pause. Two licensed clinicians - a nurse and a pharmacist or physician - must confirm the patient’s full name and date of birth using two identifiers. They must look at the patient, not just the wristband. They must read the drug label out loud. They must confirm the treatment plan matches the order.
Some nurses complain it adds 7 to 10 minutes per patient. But in facilities that implemented this fully, near-miss errors dropped by 52%. One oncology center in Wisconsin saw zero wrong-drug incidents for 14 months after enforcing the rule. The time cost is real. But the cost of a mistake? A child’s death. A mother’s liver failure. A life ruined.

Managing Cytokine Release Syndrome - The Silent Killer
Immunotherapy drugs like CAR-T cells and bispecific antibodies are revolutionizing cancer treatment. But they come with a dangerous side effect: cytokine release syndrome (CRS). This is when the immune system goes into overdrive, flooding the body with inflammatory proteins. Symptoms? Fever, low blood pressure, trouble breathing. In severe cases, it can lead to organ failure and death.
Between 2018 and 2022, CRS cases tripled. Mortality rates hit 12-15% when not treated properly. That’s why the 2024 standards now require every facility to have a CRS response plan ready. That means having tocilizumab and corticosteroids on hand. That means staff trained to recognize early signs - even before the patient feels sick. That means monitoring vital signs every 15 minutes during the first hour of infusion.
One hospital in Texas didn’t have a CRS protocol. A patient on a new immunotherapy drug developed a high fever and dropped blood pressure. Staff thought it was a viral infection. By the time they realized it was CRS, it was too late. The patient died. That’s not an anomaly. It’s preventable.
Home Chemotherapy: A Hidden Risk Zone
More than 40% of chemotherapy now happens at home. Patients get infusions through portable pumps. They take oral drugs. But most caregivers aren’t trained nurses. They’re spouses, children, aging parents. And they’re often left alone with the instructions.
The American Cancer Society found that 22% of home incidents involve improper disposal of waste - syringes, empty vials, even used tissues. Seventeen percent involve spills handled with paper towels and bleach. That’s not enough. Chemotherapy waste stays toxic for days. Bodily fluids - urine, vomit, sweat - can contain active drug for 48 to 72 hours after treatment. You need special spill kits. You need sealed containers. You need to flush toilets twice. You need to wash linens separately.
And yet, 65% of caregivers say they feel unprepared. They don’t know what to do if a vial breaks. They don’t know how to store drugs safely away from kids or pets. Facilities that use the ASCO-developed “Chemotherapy Safety at Home” toolkit - with clear videos, checklists, and 24/7 phone support - saw a 41% drop in caregiver safety concerns. That’s not magic. That’s proper support.

The Hidden Costs of Cutting Corners
Full compliance with the 2024 standards isn’t cheap. A medium-sized oncology clinic needs $22,000 to $35,000 in facility upgrades - ventilation systems, CSTDs (closed-system transfer devices), dedicated prep rooms. Staff training runs $8,500 to $12,000. Annual PPE and waste disposal costs $4,200 to $6,800. Electronic health record systems need custom builds to support the four-step verification - another $15,000 to $40,000.
But the cost of not doing it? Higher. Facilities with full compliance see 63% fewer medication errors and 78% fewer occupational exposures. OSHA issued 142 citations for hazardous drug violations in 2022 - average fine: $14,250 per violation. Medicare paid $147 million to $220 million in 2023 for preventable complications from chemotherapy errors. That’s not just money. It’s lives.
And it’s not just about money. It’s about trust. When a nurse walks into a room knowing the system has her back - the right gloves, the right checks, the right backup - she can focus on the patient. Not on whether she’ll get sick next week.
What’s Next? AI, Certification, and Equity
The future is coming fast. By 2026, a national certification for chemotherapy handlers may be required. AI-powered verification systems are being piloted at 12 top cancer centers - cameras and sensors that confirm patient identity, drug label, and dose without human error. These systems could cut the time of the fourth verification from 10 minutes to 30 seconds.
But there’s a dark side. Rural clinics can’t afford CSTDs or new EHRs. One study found 43% of rural programs can’t meet the full safety standards. That means patients in small towns may be getting the same drugs - but with less protection. That’s not equity. That’s a safety gap.
What’s clear? Chemotherapy safety isn’t optional. It’s not a suggestion. It’s the law. It’s the standard. And every person involved - from the oncologist to the caregiver cleaning up a spill - has a role. Skip a step, and you’re not saving time. You’re risking everything.
What PPE is required for handling chemotherapy drugs?
You must wear chemotherapy-tested double gloves, an impermeable gown, eye protection if splashing is possible, and a mask or respirator if aerosols or spills are likely. All PPE must be disposed of after use and treated as hazardous waste. Regular gloves or lab coats are not sufficient.
What is the fourth verification step in chemotherapy administration?
The fourth verification is a final check performed at the patient’s bedside, just before infusion begins. Two licensed clinicians must independently confirm the patient’s full name and date of birth using two identifiers, verify the drug, dose, route, and timing against the prescription, and visually confirm the patient’s identity. This step became mandatory in 2024 to reduce patient identification errors.
Why is cytokine release syndrome (CRS) now part of chemotherapy safety standards?
CRS is a life-threatening immune reaction triggered by newer immunotherapies like CAR-T and bispecific antibodies. Between 2018 and 2022, CRS cases tripled, with mortality rates of 12-15% when not treated immediately. The 2024 standards now require facilities to have antidotes like tocilizumab on hand and staff trained to recognize and respond to CRS symptoms within minutes.
Can chemotherapy be safely administered at home?
Yes, but only with proper training and equipment. Caregivers must use chemotherapy spill kits, store drugs in child-proof containers, wear gloves when handling vials or bodily fluids, and dispose of waste in approved hazardous containers. Bodily fluids remain toxic for 48-72 hours. Facilities using the ASCO “Chemotherapy Safety at Home” toolkit report 41% fewer safety concerns from caregivers.
How often should staff be trained on chemotherapy safety?
Staff must complete 8-12 hours of initial certification, including a written exam (minimum 85% score) and a practical skills demonstration. Annual 4-hour refresher training is mandatory. Competency must be reassessed every year, not just attended.
What happens if a chemotherapy spill occurs?
Use a certified chemotherapy spill kit - never paper towels or bleach. Contain the spill, wear full PPE, wipe up with absorbent pads, place all contaminated materials in a hazardous waste container, and decontaminate surfaces with a specialized cleaner. Report the incident immediately. Even small spills can lead to long-term exposure if not handled correctly.
Are there differences between U.S. and international chemotherapy safety standards?
Yes. The U.S. follows ASCO/ONS standards, which emphasize the four-step verification and PPE. The European Society for Medical Oncology (ESMO) focuses more on engineering controls like closed-system transfer devices (CSTDs). NIOSH categorizes drugs into five risk levels, guiding handling protocols. While core safety goals are similar, implementation details vary by region and resources.
Why do some nurses say the fourth verification step doesn’t reduce errors?
In facilities where the fourth verification is done as a formality - rushed, without proper communication, or without true double-checking - it won’t work. The step only reduces errors when it’s done correctly: two people, two identifiers, verbal confirmation, and full attention. If the system is overloaded or culture doesn’t support safety, the step becomes a checkbox, not a safeguard.
What You Can Do - Even If You’re Not a Professional
If you’re a patient or caregiver, ask these questions:
- Do you use double gloves and impermeable gowns when preparing my drug?
- Will two staff members verify my identity before I get the infusion?
- Do you have a plan if I have a reaction like high fever or trouble breathing?
- Do you give caregivers a spill kit and clear instructions for home use?
If the answer is no to any of these, push for better. You’re not being difficult. You’re protecting your life - and the lives of the people caring for you.
Gerald Nauschnegg
November 22, 2025 AT 21:55I saw a nurse spill chemo on the floor last week and she just wiped it with a paper towel like it was coffee. No joke. Then she went to hug her kid. I asked her if she knew the drug stays active for 72 hours and she said, 'We're just too busy to care.' That's not negligence, that's a death sentence waiting to happen.
Palanivelu Sivanathan
November 23, 2025 AT 03:12Listen… we’re all just atoms dancing in the void, right? But somehow, in this chaotic universe, we’ve created a system where a single glove tear can end a life… or two… or ten. It’s not about protocols. It’s about the arrogance of human control. We think we can tame poison with checklists. But poison doesn’t care about your checklist. It just waits. And watches. And lingers… on the toy your toddler picks up.
Joanne Rencher
November 24, 2025 AT 00:05So we need FOUR people to check a drug? And special gloves? And hazardous waste bins? Wow. What a shocker. Meanwhile, my cousin got chemo in a trailer park clinic with reused gloves and a clipboard. Guess what? She’s alive. Maybe we’re overcomplicating this because we’re too lazy to just… care?
Erik van Hees
November 25, 2025 AT 13:11You’re all missing the real issue. The 2024 guidelines are outdated already. NIOSH just released a new permeation study showing that even 'chemo-tested' gloves fail in under 12 minutes with newer antibody-drug conjugates. And no one’s talking about the fact that the fourth verification step is useless if the staff are exhausted from 12-hour shifts and the EHR system crashes every time you try to log in. We’re applying Band-Aids to a hemorrhage. The real solution? AI-driven verification with facial recognition and barcode scanning - already proven in 12 top centers. But nope, we’re still arguing over gloves while kids die.
Cristy Magdalena
November 25, 2025 AT 21:24It’s not just about safety. It’s about dignity. When a nurse wears double gloves and a gown, she’s not just protecting herself-she’s saying, 'I am not expendable.' And when a caregiver is given a spill kit and a 24/7 hotline, she’s being told, 'Your life matters too.' But in so many places, they’re treated like disposable tools. And that’s the real cancer here-the belief that some lives are worth less than others.
Adrianna Alfano
November 26, 2025 AT 09:51i just had my mom go through chemo at home and honestly no one told us anything. we used paper towels to clean up a spilled vial because the nurse said 'it's fine, just wash it'... i cried for three days after reading this. i had no idea my hands were toxic for days. we need these kits. we need training. i'm not a nurse, i'm a college student. why is this on me? i'm so mad.
Casey Lyn Keller
November 28, 2025 AT 08:13Let me guess. The same people pushing these expensive protocols are the ones who own the PPE companies. And the AI verification startups? Probably funded by big pharma. Meanwhile, rural clinics are getting told to 'just do better' while their budget got cut by 40%. This isn’t safety. It’s profit dressed up as policy.
Jessica Ainscough
November 28, 2025 AT 18:13I’ve been an oncology nurse for 14 years. I’ve seen the good, the bad, and the terrifying. The fourth verification? It saved my patient last month. She had the same name as someone else. The system almost mixed them up. We paused. We checked. We caught it. It’s not about slowing down. It’s about making sure the person getting the drug is the person who needs it. I’m tired. But I’ll keep doing it. Because someone’s life depends on it.
May .
November 29, 2025 AT 14:48